Device selection for patients with cancer
Version 1: January 2024
Device selection is the timely, informed, and proactive process of choosing the most appropriate vascular access device (VAD) for the individual patient and prescribed therapy (1). The aim is to insert one device for an episode of care with no complications. Appropriate device selection and insertion reduces the risk of damaged caused by repeated access and preserves vein health for current and future use (1). Device selection is influenced by patient, device, therapy, and workplace related factors (2). This topic will detail the findings from current literature for device selection for patients with cancer.
TERMINOLOGY
Common language facilitates communication between professional and organisations, the translation of evidence into clinical practice, data sharing, and research. CNSA, eviQ and eviQ Education are leading vascular access management and education in cancer care in Australia utilizing common, contemporary terminology (Table 1).
Table 1: CVAD terminology
INTRODUCTION
Successful vascular access outcomes begin with appropriate device selection and maximising first insertion success to reduce VAD related complication, premature removal and reinsertion rates. This also minimises patient distress and pain and facilitates patient safety (3-5). This process includes -
- Consultation: with appropriate healthcare teams e.g. vascular access, renal, infectious diseases, pharmacy (2, 3, 6-10)
- Device: insertion of the smallest size catheter required for the therapy in the largest vein including peripheral intravenous cannulas (10), fewest number of lumens and least invasive VAD for the required therapy (11-13)
- Vein: insertion into the largest vein in the healthiest tissues avoiding injured areas (3, 14), avoiding areas of flexion (15)
- Timeframe: at earliest opportunity e.g. 24-48 hours after determining need for intravenous therapy
- Evaluation: with daily assessment for continued needs of the VAD (7, 12, 16)
The device selection process is standardised via algorithms documented in clinical procedures or electronic medical record order sets (5, 17-19), or with criteria for appropriateness considering multiple clinical scenarios (2, 8). The process commonly commences with identifying the urgency of the selection (emergent or elective), then proceeds to assessment of the type of therapy (peripherally or centrally compatible) and duration (days, weeks, months, years) of therapy. If the therapy is peripherally compatible, a peripheral venous assessment is performed (1, 18).
Device selection describes core time-related, patient-related, device-related, therapy-related, and workplace-related factors (1, 2, 4, 7, 17, 20, 21) which focuses the process on the patients’ needs and minimises clinician preferences and biases (20). These factors will be detailed in the following sections.
SUMMARY OF RECOMMENDATIONS
SUMMARY OF RECOMMENDATIONS
1. Education
CNSA recommends patient, family and healthcare team education includes VAD selection considering all relevant factors that may influence the decision for adult and paediatric patients with cancer to make an informed decision (8, 9, 13, 17, 19, 22-35) including:
- Patient-related factors: diagnosis and comorbidities, venous assessment, age, current pathology, and cognition and collaboration capacity.
- Time related factors: emergent or elective
- Therapy-related factors: type (peripheral or central compatibility), number and compatibility of therapies, timing (administration speed, duration - days, weeks, months, years, frequency - intermittent, continuous), intensity, setting (acute, ambulatory, community, home), and potential adverse effects.
- Workplace-related factors: availability of a range of VADs, access to technology (ultrasound, near infrared imaging), education of the healthcare team, patient and family, inclusion of the device selection process in clinical practice and procedures, VAD selection outcomes recorded in patient health records. Potential VADs include PIVCs, midlines, PICCs, CICCs, tc-CICCs, TIVAD and high flow CVADs e.g. apheresis CICCs/TIVADs or haemodialysis CICCs.
- Evaluation: including daily assessment for continued need of the VAD
GRADE Ib
2. Time related factors
CNSA recommends selection of the most appropriate vascular access device for elective insertion is accomplished within 24-48 hours from determining the need for intravenous therapy. Emergent VAD selection is determined by the medical needs of the patient (1-3, 5, 17, 18, 21, 23, 36).
GRADE Ib
3. Patient related factors
CNSA recommends patient-related factors are considered during the device selection process including:
- 3.1 Patient choice including values, experiences and preferences, capacity for collaboration for VAD maintenance, social relationships and support, and health literacy (7, 25, 30, 32, 33, 35, 37-81).
GRADE III
- 3.2 Diagnoses, comorbidities and the associated pathophysiology including:
- Acute or chronic renal failure (2, 3, 6-9) GRADE V
- Oncology or malignant haematology disease (19, 20, 56, 58, 82-85). GRADE III
- Non-malignant haematological disorders e.g. haemophilia (20, 86). GRADE V
- Disorders of the gastrointestinal tract, conditions or surgery that may require short or long-term parenteral nutrition (2, 8, 11, 87-92). GRADE Ib
- History of thrombosis (2, 24, 93, 94). GRADE Ib
- Diabetes (7, 95). GRADE IV
- Increased or decreased body mass index or body habitus (7, 42, 85, 96, 97). GRADE IV
3.3 Venous assessment including:
- Peripheral and central veins (2, 7, 17, 22, 98). GRADE Ib
- Number, visibility, palpability, and condition of veins (19, 99-110). GRADE IV
- Vein size: vein diameter to calculate catheter to vein ratio occupying <45% for adult patients diagnosed with cancer and <40% for paediatric patients (10, 13, 111). GRADE Ib
- Using palpation or visual inspection (19). GRADE V
- Using assistive visual technology, for example ultrasound, near infrared (1, 2, 63, 95, 112-119). GRADE IV
3.4 Age including paediatric patients with congenital or age related diagnoses (21). GRADE V
3.5 Pathology results including full blood examination, coagulation profile and renal function (2, 3, 6-9). GRADE Ib
4. Therapy related factors
CNSA recommends therapy-related factors are considered during the device selection process including:
4.1 Type of therapy including:
- Infusate/s characteristics (peripheral or centrally compatibility) including osmolarity, pH, viscosity, vesicant, irritant, or vasoactivity of the infusate (1, 2, 7, 18, 31). GRADE Ib
- Number and compatibility of infusates (21). GRADE V
- Consult pharmacist in the healthcare team as required (120). GRADE IV
4.2 Time related factors including:
- Intensity e.g. (for autologous or allogeneic bone marrow transplant) (1, 2, 6, 7, 121). GRADE Ib
- Frequency GRADE CC
- Speed GRADE CC
- Duration GRADE CC
4.3 Setting including potential transition between inpatient, outpatient, community or home, e.g. home parenteral nutrition, infusional chemotherapy or antibiotics (7, 16, 20). GRADE V
4.4 Potential side effects including bleeding, clotting, inflammation and infection (7). GRADE V
Refer to eviQ protocols for information about central or peripheral compatibility of SACTs for haematological cancers and solid tumour cancers.
5. Device related factors
CNSA recommends all types of VADs available within a workplace are considered during the device selection process potentially including:
5.1 Peripheral VADs
- 5.1.1 Standard (short) PIVCs are appropriate for:
- administration of peripherally compatible infusates (2, 7)
- for short duration (Table 3, Table 4)
- patients with good veins (2, 17, 20) or DIVA (2)
- preferred VAD for patients with haemophilia (86)
- peripherally compatible chemotherapy < 3 months (2)
- can be used for apheresis procedures (115)
- GRADE Ib
- 5.1.2 Ultrasound guided (USG) PIVCs:
- e.g. up to 7-8cm (5, 122)
- non vesicants of dwell time of up to 28 days (2, 6, 7, 17, 20) (Table 3)
- patients with DIVA (2, 7, 108)
- alternative for regularly replaced standard PIVCs in paediatrics (123)
- GRADE Ib
- 5.1.3 PIVCs with additional features:
- retractable coiled guidewire for insertion (124).
- wings and injection port (open port with no needleless connector) (125) or wings, injection port and instant blood flashback (126-128). NOTE: Analyses of the dwell time were not included.
- different materials (129, 130)
- cannula with outlet via holes on the side e.g. for computed tomography (131)
- GRADE I
- 5.1.4 Midlines
- suitable only for peripherally compatible infusates only e.g. green coded infusates
- shorter term duration – days to weeks (2, 108, 132-136)
- potential to reduce insertion of more invasive devices e.g. PICCs (17, 22, 137, 138) or repeated venepuncture over short term e.g. days to weeks (139)
- avoid use with patients with a history of thrombosis, hypercoagulability (7).
- GRADE Ib
5.2 CVADs
- 5.2.1 PICC
- inpatient, ambulatory/outpatient or home settings (2)
- paediatrics, adults to palliative care, including end of life care (2, 65, 92, 140-143)
- for home parenteral nutrition (87), vesicants or inotropes (108), chemotherapy ≥3 months (2), haemodynamic monitoring for critically unwell patients (2, 94), autologous or allogeneic transplant (140)
- tunnelled PICC: optimal exit site via tunnelling the catheter from the appropriate venepuncture site (for catheter to vein ratio) to area of comfort for the patient and flat area for dressing management reduces complications (144). Alternately, for patients with chest pathology impeding upper body placement, an alternative is PICC insertion via the femoral vein using intracavity ECG for optimal tip position, in the inferior vena cava above the level of the diaphragm (145)
- complications, or vein size may vary according to right, left side or hand dominance (146, 147) with less complications on right side PICCs (59, 148)
- GRADE Ib
- 5.2.2 TIVAD
- inpatient, ambulatory/outpatient or home settings
- longer term therapies (2) e.g. chemotherapy and preferred by patients for chemotherapy (35, 45, 54, 58, 74, 75, 83, 149-155) or high flow procedures e.g. apheresis (156, 157)
- traditional site is anterior upper chest, alternative sites in paediatric and adult patients (158, 159): arm-TIVAD (7, 32, 160-165) e.g. for patients with breast cancer (50, 166-168), lateral chest in deltopectoral groove (169), trapezius region (170) or via different veins (171, 172) e.g. internal jugular, subclavian veins (173-175)
- insertion with appropriate sedation, pain relief or distraction therapy, e.g. virtual reality (176) during and possibly after the insertion procedure (54, 71, 177-181)
- GRADE Ib
- 5.2.3 tc-CICC
- can be used for inpatient, ambulatory/outpatient or home settings
- longer term therapies e.g. months/years, for SACTs, commonly via internal jugular, or when PICC is not feasible (1, 2, 182, 183)
- GRADE Ib
- 5.2.3 CICC / FICC
- inpatient use (7)
- dhort term use (1, 2) unless tunnelled
- e.g. parenteral nutrition, vesicant, inotropes (108), apheresis procedures (184)
- GRADE Ib
6. Workplace factors
CNSA recommends the workplace facilitates implementation of a device selection strategy within clinical practice by:
- 6.1 Education of the healthcare team including the patient and family (19, 185). GRADE IV
- 6.2 Documentation of a device selection algorithm within patient health records and procedures. GRADE CC
- 6.3 Vascular access teams (VATs) if available, provide expert advice in the device selection process (9, 20, 22, 24, 36, 75, 117, 186-188). GRADE IV
- 6.4 Availability of a range of VAD types and appropriately educated and competent clinicians for insertion (7, 9). GRADE V
7. Evaluation
CNSA recommends the standardised daily assessment of a VAD via a validated tool, for example I-DECIDED to evaluate the VAD selection and the continued use of the VAD (1, 12, 18, 189).
GRADE V
1. EDUCATION
1.1 Summary of evidence
Education of the healthcare team including the patient and family is a cornerstone of clinical practice.
Education about a standardised, evidence-based approach to device selection for the individual patient and required prescribed therapy is critical (31, 71). An algorithm or decision making tree included in workplace procedures provides a consistent approach for implementation by all members of the healthcare team (8, 9, 17, 22-28) and considers cancer related therapies (19).
1.2 Practice Recommendation
CNSA recommends the patient, family and healthcare team are educated about appropriate vascular access device selection, considering all relevant factors that may influence the decision including systemic anticancer therapies (8, 9, 13, 17, 19, 22-31, 35).
GRADE Ib
2. TIME-RELATED FACTORS
2.1 Summary of evidence
The emergent or elective need for vascular access initially determines the most appropriate VAD (1, 2, 21). Peripheral intravenous cannulas, intraosseous or centrally inserted central catheters can be inserted in medical emergencies (1, 2, 17, 18, 23). Time to make an informed decision for elective insertions enables consultation and consideration of key factors which potentially impact the shared decision-making process. This should be accomplished within 24-48 hours from determining the need for intravenous therapy (3).
2.2 Practice Recommendation
CNSA recommends selection of the most appropriate vascular access device for elective insertion is accomplished within 24-48 hours from determining the need for intravenous therapy. Emergent VAD selection is determined by the medical needs of the patient (1-3, 5, 17, 18, 21, 23, 36).
GRADE Ib
3. PATIENT-RELATED FACTORS
3.1 Summary of evidence
Patient related factors which impact the suitability of one type of VAD compared to another include:
A. Patient choice, cognition, collaboration
A patient’s values, experiences and preferences are fundamentally important to consider during the device selection process (25, 30, 32, 33, 35, 37-79, 83). Other factors include their capacity for collaboration for VAD maintenance, social relationships and support, and health literacy (7, 80, 190).
B. Diagnosis
Consideration of a patient’s diagnosis, comorbidities and the associated pathophysiology influence the device selection process and inform which members of the healthcare team need to be consulted (for example nephrology, oncology/haematology, infectious diseases) include:
- acute and chronic renal failure e.g. Stage 3b or greater (2, 3, 6-9) will determine if veins will be required for future haemodialysis and exclude the use of peripheral veins
- oncology or malignant haematology disease (19, 20, 56, 58, 82-85)
- non-malignant haematological disorders e.g. haemophilia (20, 86)
- disorders of the gastrointestinal tract, conditions or surgery that may require short or long-term parenteral nutrition (2, 8, 11, 87-92).
- history of thrombosis (2, 24, 93, 94).
- diabetes with associated delayed healing and potential increased risk of infection (7, 23, 95).
- increased or decreased body mass index or body habitus (7, 42, 85, 96, 97).
- tracheostomy adjacent to a upper chest CVAD potentially increasing the risk of infection (7).
C. Venous assessment
Assessment of a patient’s peripheral and central veins is critical for device selection (2, 7, 17, 22, 98) including the number, visibility, palpability, and condition of veins (19, 99-110). Vein size, that is the diameter of the vein, is used when calculating the catheter to vein ratio (CVR): the proportion of the vein that the catheter or cannula occupies. This is relevant for PIVC and CVAD insertion (10) with CVR< 45% for adult patients diagnosed with cancer (111) and CVR<40% recommended for paediatric patients (13). Assessment can be graded, for example according to the number of veins (1, 100, 104), with or without a torniquet (107, 191), using different techniques e.g. assistive visual technology (ultrasound, NIR) (1, 2, 63, 95, 112-119), palpation, visual inspection (19) or description using key terms, which may be open to interpretation (18) e.g. excellent, good, sufficient, poor (17, 22, 103).
Venous assessment facilitates early identification of patients with difficult intravenous access (DIVA) to enable the selection of the most appropriate device from the outset (2, 18) which may include long PIVCs into deeper, larger veins via ultrasound, midlines (138) or CVADs (1). Short PIVC are avoided (7).
D. Age
Additional factors that require consideration when selecting the most appropriate VAD for paediatric patients include congenital heart disease or surgery, abnormal vascular anatomy, skin conditions e.g. epidermolysis bullosa (6, 21).
E. Pathology
Assessment of a patient’s blood pathology results include: (7)
- Coagulation profile and platelets for implanted and tunnelled VADs
- Full blood count e.g. neutrophils for the risk of infection
- Renal function e.g. acute or chronic dysfunction as identified earlier (2, 3, 6-9)
3.2 Practice Recommendation
CNSA recommends patient-related factors are considered during the device selection process including:
- 3.2.1 Patient choice including values, experiences and preferences, capacity for collaboration for VAD maintenance, social relationships and support, and health literacy (7, 25, 30, 32, 33, 35, 37-81).
GRADE III
- 3.2.2 Diagnoses, comorbidities and the associated pathophysiology including:
- Acute or chronic renal failure (2, 3, 6-9) GRADE V
- Oncology or malignant haematology disease (19, 20, 56, 58, 82-85). GRADE III
- Non-malignant haematological disorders e.g. haemophilia (20, 86). GRADE V
- Disorders of the gastrointestinal tract, conditions or surgery that may require short or long-term parenteral nutrition (2, 8, 11, 87-92). GRADE Ib
- History of thrombosis (2, 24, 93, 94). GRADE Ib
- Diabetes (7, 95). GRADE IV
- Increased or decreased body mass index or body habitus (7, 42, 85, 96, 97). GRADE IV
- 3.2.3 Venous assessment including:
- Peripheral and central veins (2, 7, 17, 22, 98). GRADE Ib
- Number, visibility, palpability, and condition of veins (19, 99-110). GRADE IV
- Vein size: vein diameter to calculate catheter to vein ratio occupying <45% for adult patients diagnosed with cancer and <40% for paediatric patients (10, 13, 111). GRADE Ib
- Using palpation or visual inspection (19). GRADE V
- Using assistive visual technology, for example ultrasound, near infrared (1, 2, 63, 95, 112-119). GRADE IV
- 3.2.4 Age including paediatric patients with congenital or age related diagnoses (21). GRADE V
- 3.2.5 Pathology results including full blood examination, coagulation profile and renal function (2, 3, 6-9). GRADE Ib
4. THERAPY-RELATED FACTORS
4.1 Summary of evidence
Therapy-related factors that impact the appropriateness of one type of VAD compared to another include:
A. Type of therapy
Firstly, establish if the infusates are peripherally or centrally compatible. Some infusates are not safe for administration through peripheral veins as they can damage the lining of the vein and require central veins with large diameters and adequate haemodilution. Infusate characteristics that need to be considered are: osmolarity, pH, viscosity, vesicant, irritant, or vasoactivity of the infusate (1, 2, 7, 18, 31) (Table 2).
The number and compatibility of concurrent infusates also needs to be considered for type of VAD and the number of lumens required e.g. methotrexate, leucovorin are administered on a separate lumen (21). Collaborate with the pharmacist in the healthcare team during the device selection process as required (120).
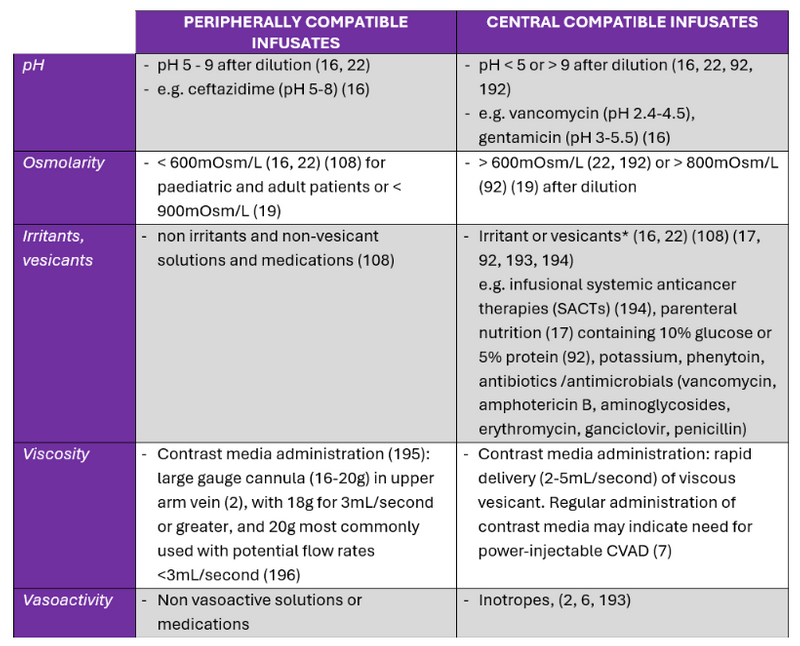
Table 2. Summary of literature: infusate characteristics for administration in peripheral and central veins.
*Summary of evidence and not recommendations for practice
Published tools can colour code infusates according to the risk of damage, irritation or thrombosis of the vein, for example red, yellow or green which use a traffic light concept. Green or lower risk infusates are compatible for peripheral administration, peripheral administration with caution is recommended for the yellow coded or intermediate risk category and central administration is recommended for red or high risk category infusates (19, 21). Infusates can be categorised according to their vesicant, irritant, or non-vesicant properties (19).
B. Time-related factors
The speed of administration (2.5mL per hour to >100 mL /min for fluid resuscitation), duration of therapy (days, weeks, months, ongoing), frequency (intermittent – daily or 1-4 weekly, or continuously), intensity (for autologous or allogeneic bone marrow transplant) will determine whether peripheral or central administration is appropriate (1, 2, 6, 7, 121) e.g. large diameter VADs with multiple lumens into large veins for rapid fluid resuscitation, intermittent or peripherally compatible therapies via a PIVC or midline, continuous or intense therapies via a CVAD.
Recommendations for VADs according to the duration and type of therapy in the literature are summarised in Table 3 and Table 4.
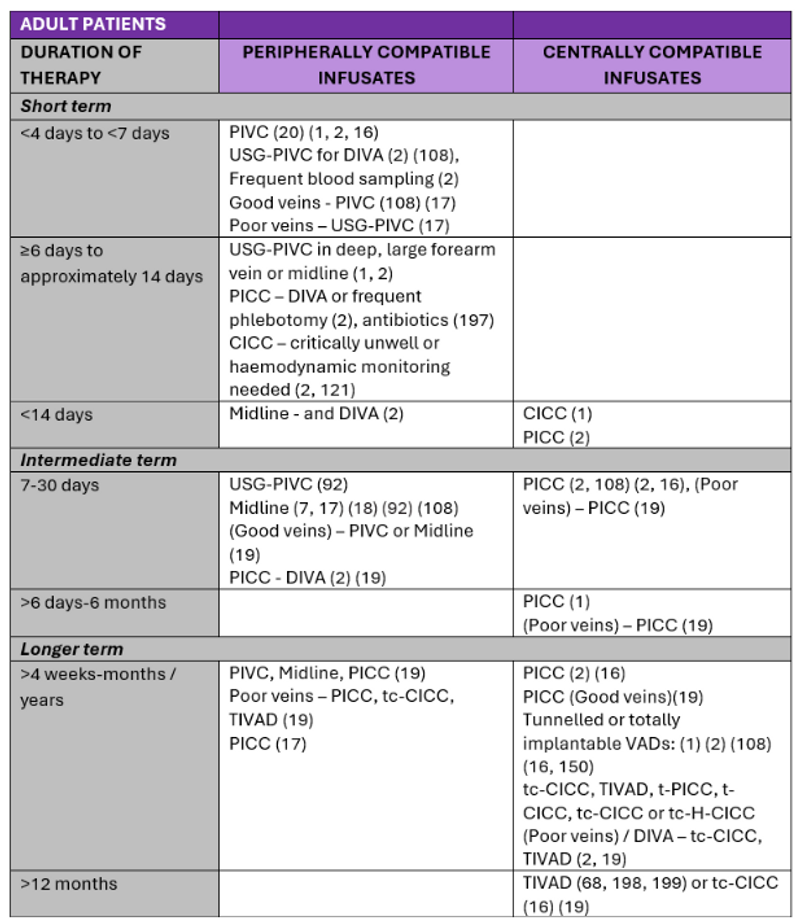
Table 3. Summary of literature: VADs for administration of peripherally and centrally compatible infusates in adult patients
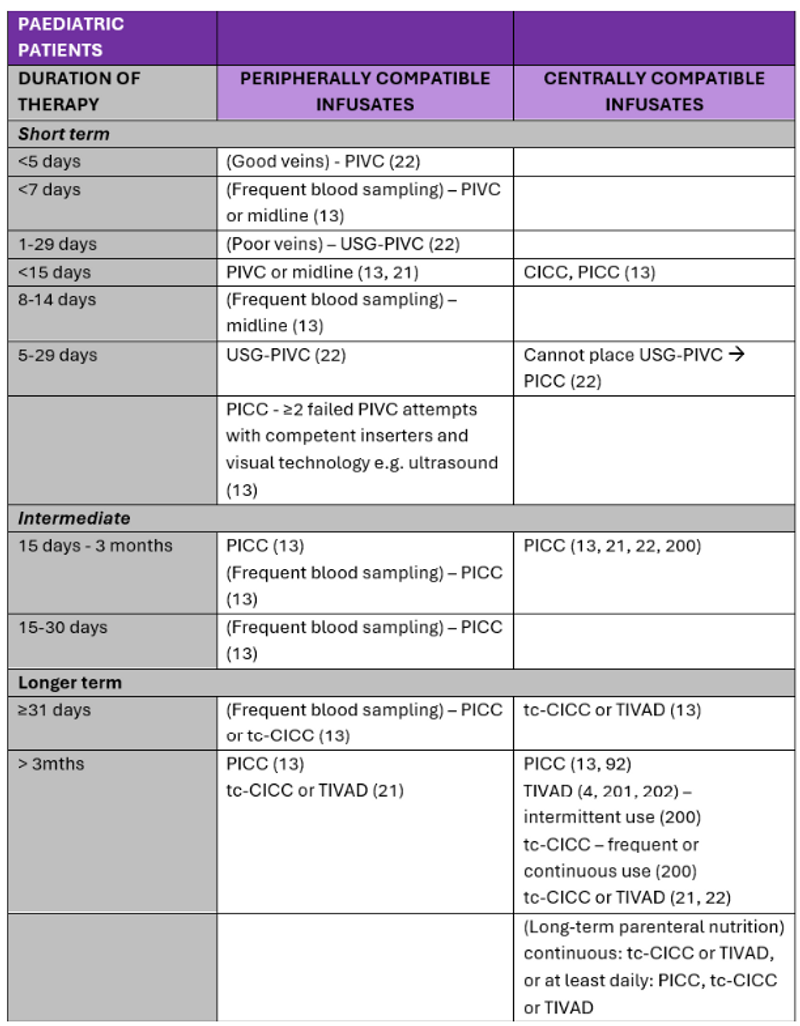
Table 4. Summary of literature: VADs for administration of peripherally and centrally compatible infusates in paediatric patients
C. Setting
Patients with cancer can transition between acute, ambulatory/outpatient, community and home settings during the administration of prescribed therapies. Consideration of therapy continuing on an outpatient basis, e.g. home parenteral nutrition, infusional chemotherapy or antibiotics needs to be considered during the device selection process (7, 16, 20).
D. Potential side effects of therapy
Bleeding, clotting, inflammation and infection risks associated with the adverse effects of prescribed therapy are considered during the device selection process (7) e.g. risk of considerable bruising with insertion of a tunnelled or subcutaneous CVAD or repeated needle access into a TIVAD in a thrombocytopenic or coagulopathic patient, infection risk of CVAD insertion during periods of profound neutropenia, or the infection risk between TIVADs and external catheters e.g. PICC, CICCs.
4.2 Practice Recommendation
CNSA recommends therapy-related factors are considered during the device selection process including:
- 4.2.1 Type of therapy including:
- Infusate/s characteristics (peripheral or centrally compatibility) including osmolarity, pH, viscosity, vesicant, irritant, or vasoactivity of the infusate (1, 2, 7, 18, 31). GRADE Ib
- Number and compatibility of infusates (21). GRADE V
- Consult pharmacist in the healthcare team as required (120). GRADE IV
- 4.2.2 Time related factors including:
- Intensity e.g. (for autologous or allogeneic bone marrow transplant) (1, 2, 6, 7, 121). GRADE Ib
- Frequency GRADE CC
- Speed GRADE CC
- Duration GRADE CC
- 4.2.3 Setting including potential transition between inpatient, outpatient, community or home, e.g. home parenteral nutrition, infusional chemotherapy or antibiotics (7, 16, 20). GRADE V
- 4.2.4 Potential side effects including bleeding, clotting, inflammation and infection (7). GRADE V
Refer to eviQ protocols for information about central or peripheral compatibility of SACTs for haematological cancers and solid tumour cancers.
5. DEVICE-RELATED FACTORS
5.1 Summary of evidence
PIVC
Clinical expertise is critical to determine whether a PIVC is appropriate (20). Carr and colleagues (2017) discussed the 80% rule: PIVCs are not inserted unless the clinician is 80% sure it will be used. The aim was to reduce the rate of PIVCs that were inserted and not used (203, 204). This was validated in an implementation study by Hawkins and colleagues (2018) increasing the insertion of clinically indicated PIVCs in the emergency department (205).
Standard (short) PIVCs are appropriate for:
- administration of peripherally compatible infusates (2, 7)
- for short duration (Table 3, Table 4)
- patients with good veins (2, 17, 20) or DIVA (2)
- and preferred VAD for patients with haemophilia (86)
- peripherally compatible chemotherapy < 3 months (2)
- conflicting information about blood sampling - frequent blood sampling ≤ 5 days (2) or recommended not for blood sampling (20)
- can be used for apheresis procedures (115)
Ultrasound guided (USG) PIVC
- e.g. up to 7-8cm (5, 122)
- non vesicants of dwell time of up to 28 days (2, 6, 7, 17, 20) (Table 3)
- patients with DIVA (2, 7, 108)
- alternative for regularly replaced and multiple standard PIVCs in paediatrics (123)
PIVC with additional features:
- retractable coiled guidewire for insertion: two studies investigating PIVCs with retractable coiled guidewires had conflicting results –
- a randomised controlled study (RCT) of 248 surgical adolescent participants recorded considerably greater first time success rates and more patients completed therapy with PIVCs with guidewires compared to standard PIVCs (124)
- a RCT in interventional radiology demonstrated no difference between the standard cannula and one with a retractable coiled guidewire, however this could be explained by the inserters having extensive experience with standard cannula, which additionally had blood control (206)
- wings and injection port (open port with no needleless connector): superior handling rating for insertion in large RCT compared to standard PIVC (125). Or improved first insertion success with a cannula with wings, injection port and instant blood flashback (126, 127) which facilitated more rapid feedback about blood return (128). Analysis of the dwell time was not included.
- different materials (129) and additional to this review, a systematic review of different materials (130)
- cannula with outlet via holes on the side e.g. for computed tomography (131)
Midline
Midlines are an alternative to the USG-PIVCs in deep veins, similarly they are used for the administration of peripherally compatible infusates only and are approximately 8-20cm in length (116). Midlines can be:
- inserted to potentially reduce the use of more invasive devices e.g. PICCs (17, 22, 137, 138)
- for shorter term duration – days to weeks (2, 108, 132-136)
- reduces repeated venepuncture over the short term e.g. days to weeks (139)
- high risk patients requiring more than five days of therapy e.g. patients with pneumonia, undergoing surgery, gastrointestinal bleed, respiratory disease (207)
- for the paediatric patient, the distal tip of midlines do not terminate in joint spaces, enter the central circulation, or enter the pelvic region (22)
Avoid the use of midlines for patients with a history of thrombosis, hypercoagulability related to the risk of thrombosis or renal disease for potential future haemodialysis (7).
There is no evidence for the safe administration of SACTs via a midline. The location of the midline catheter tip in a deeper vein compared to a PIVC (in a superficial vein) may lead to a delay in detecting an extravasation and potentially more extensive extravasation injury or injury of anatomical structures, for example arteries and nerves (139). Also cautious use of midlines in critical care settings for potential short-term administration of irritants in emergent situations until CVAD insertion is achieved (208).
CVADs
- CVAD characteristics include:
- single or multiple lumens
- standard or power-injectable
- non-valved or valved (distal or proximal) (209)
- tunnelled or non-tunnelled
- standard or cuffed catheters
- standard or coated e.g. anti-infective or antithrombotic coating (202)
- totally implantable venous access devices – chest, arm
- large diameter catheters for apheresis and haemodialysis procedures
CVADs are suitable for administration of peripherally compatible infusates and centrally compatible infusates, as noted in the section above (Table 3 and 4). CVAD uses include:
- PICC
- can be used for inpatient, ambulatory/outpatient or home settings
- paediatrics, adults to palliative care, including end of life care (2, 65, 92, 140-143)
- home parenteral nutrition (87), vesicants or inotropes (108), chemotherapy ≥3 months (2), haemodynamic monitoring for critically unwell patients (2, 94), autologous or allogeneic transplant (140)
- optimal exit site location via tunnelling the catheter from the appropriate venepuncture site (for catheter to vein ratio) to area of comfort for the patient and flat area for dressing management reduces complications (144). Alternately, for patients with chest pathology impeding upper body placement, an alternative is PICC insertion via the femoral vein using intracavity ECG for optimal tip position, in the inferior vena cava above the level of the diaphragm (145)
- complications, or vein size may vary according to right, left side or hand dominance (146, 147) with less complications on right sided PICCs (59, 148)
- TIVAD
- can be used for inpatient, ambulatory/outpatient or home settings
- for administration of longer term therapies (Table 3), e.g. chemotherapy and preferred by patients for chemotherapy (35, 45, 54, 58, 74, 75, 83, 149-155) or high flow procedures e.g. apheresis (156, 157)
- traditional site is on anterior upper chest, with alternative sites in paediatric and adult patients (158, 159): arm-TIVAD (7, 32, 160-165) e.g. for patients with breast cancer (50, 166-168), lateral chest in deltopectoral groove (169), trapezius region (170) or via different veins (171, 172) e.g. internal jugular, subclavian veins (173-175)
- with appropriate sedation, pain relief or distraction therapy, e.g. virtual reality (176) during and possibly after the insertion procedure (54, 71, 177-181)
- tc-CICC
- can be used for inpatient, ambulatory/outpatient or home settings
- for administration of longer term therapies e.g. months/years, for SACTs, commonly via internal jugular, or when PICC is not feasible (1, 2, 182, 183)
- CICC / FICC
- inpatient use (7)
- short term use (1)
- e.g. parenteral nutrition, vesicant, inotropes (108), apheresis procedures (184)
For patients with haemophilia, PIVCs are preferred compared to CVAD (86). CVADs are inserted if the patient has no active infections including severe dental problems, has no history of CVAD related thrombosis, the patient and family adhere with the maintenance requirements, and removed as soon as no longer required (86).
5.2 Practice Recommendation
CNSA recommends all types of VADs available within a workplace are considered during the device selection process potentially including:
5.2.1 Peripheral VADs
- Standard (short) PIVCs are appropriate for:
- administration of peripherally compatible infusates (2, 7)
- for short duration (Table 3, Table 4)
- patients with good veins (2, 17, 20) or DIVA (2)
- preferred VAD for patients with haemophilia (86)
- peripherally compatible chemotherapy < 3 months (2)
- can be used for apheresis procedures (115)
- GRADE Ib
- Ultrasound guided (USG) PIVCs:
- e.g. up to 7-8cm (5, 122)
- non vesicants of dwell time of up to 28 days (2, 6, 7, 17, 20) (Table 3)
- patients with DIVA (2, 7, 108)
- alternative for regularly replaced standard PIVCs in paediatrics (123)
- GRADE Ib
- PIVCs with additional features:
- retractable coiled guidewire for insertion (124).
- wings and injection port (open port with no needleless connector) (125) or wings, injection port and instant blood flashback (126-128). NOTE: Analyses of the dwell time were not included.
- different materials (129, 130)
- cannula with outlet via holes on the side e.g. for computed tomography (131)
- GRADE I
- Midlines
- suitable only for peripherally compatible infusates only e.g. green coded infusates
- shorter term duration – days to weeks (2, 108, 132-136)
- potential to reduce insertion of more invasive devices e.g. PICCs (17, 22, 137, 138) or repeated venepuncture over short term e.g. days to weeks (139)
- avoid use with patients with a history of thrombosis, hypercoagulability (7).
- GRADE Ib
5.2.2 CVADs
- PICC
- inpatient, ambulatory/outpatient or home settings (2)
- paediatrics, adults to palliative care, including end of life care (2, 65, 92, 140-143)
- for home parenteral nutrition (87), vesicants or inotropes (108), chemotherapy ≥3 months (2), haemodynamic monitoring for critically unwell patients (2, 94), autologous or allogeneic transplant (140)
- tunnelled PICC: optimal exit site via tunnelling the catheter from the appropriate venepuncture site (for catheter to vein ratio) to area of comfort for the patient and flat area for dressing management reduces complications (144). Alternately, for patients with chest pathology impeding upper body placement, an alternative is PICC insertion via the femoral vein using intracavity ECG for optimal tip position, in the inferior vena cava above the level of the diaphragm (145)
- complications, or vein size may vary according to right, left side or hand dominance (146, 147) with less complications on right side PICCs (59, 148)
- GRADE Ib
- TIVAD
- inpatient, ambulatory/outpatient or home settings
- longer term therapies (2) e.g. chemotherapy and preferred by patients for chemotherapy (35, 45, 54, 58, 74, 75, 83, 149-155) or high flow procedures e.g. apheresis (156, 157)
- traditional site is anterior upper chest, alternative sites in paediatric and adult patients (158, 159): arm-TIVAD (7, 32, 160-165) e.g. for patients with breast cancer (50, 166-168), lateral chest in deltopectoral groove (169), trapezius region (170) or via different veins (171, 172) e.g. internal jugular, subclavian veins (173-175)
- insertion with appropriate sedation, pain relief or distraction therapy, e.g. virtual reality (176) during and possibly after the insertion procedure (54, 71, 177-181)
- GRADE Ib
- tc-CICC
- can be used for inpatient, ambulatory/outpatient or home settings
- longer term therapies e.g. months/years, for SACTs, commonly via internal jugular, or when PICC is not feasible (1, 2, 182, 183)
- GRADE Ib
- CICC / FICC
- inpatient use (7)
- dhort term use (1, 2) unless tunnelled
- e.g. parenteral nutrition, vesicant, inotropes (108), apheresis procedures (184)
- GRADE Ib
6. WORKPLACE-RELATED FACTORS
6.1 Summary of evidence
Several factors that potentially impact device selection process include:
- Education and procedures: As discussed earlier, availability and frequency of education of the healthcare team including the patient, and inclusion of device selection algorithms within workplace policy is key to standardised, evidence-based device selection and the different types of VADs (19, 185).
- Vascular access teams (VATs) if available, provide expert advice in the device selection process (9, 20, 22, 24, 36, 75, 117, 186-188).
- Availability of different types VADs: is required for the device selection process (7-9). Not all workplaces stock all types of devices and have trained clinicians to insert the different VADs.
6.2 Practice Recommendation
CNSA recommends the workplace facilitates implementation of a device selection strategy within clinical practice by:
- 6.2.1 Education of the healthcare team including the patient and family (19, 185). GRADE IV
- 6.2.2 Documentation of a device selection algorithm within patient health records and procedures. GRADE CC
- 6.2.3 Vascular access teams (VATs) if available, provide expert advice in the device selection process (9, 20, 22, 24, 36, 75, 117, 186-188). GRADE IV
- 6.2.4 Availability of a range of VAD types and appropriately educated and competent clinicians for insertion (7, 9). GRADE V
7. EVALUATION
7.1 Summary of evidence
Daily assessment of the continued need for a VAD, and assessment of complications via implementation of a standardised, validated tool is required, for example I-DECIDED (1, 12, 18, 189). Data collection from routine documentation in patient health records for example patient, VAD, dwell time, complications and removal can be used to evaluate vascular access outcomes and inform future quality improvement initiatives (19, 31).
7.2 Practice Recommendation
CNSA recommends the standardised daily assessment of a VAD via a validated tool, for example I-DECIDED to evaluate the VAD selection and the continued use of the VAD (1, 12, 18, 189).
GRADE V
REFERENCES
REFERENCES for Device selection page – VHP topic
1. Hallam C, Denton A, Weston V, Dunn H, Jackson T, Keeling S, et al. UK Vessel Health and Preservation (VHP) Framework: a commentary on the updated VHP 2020. Journal of infection prevention. 2021;22(4):147-55.
2. Chopra V, Flanders SA, Saint S, Woller SC, O'Grady NP, Safdar N, et al. The Michigan appropriateness guide for intravenous catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA Appropriateness Method. Annals of Internal Medicine. 2015;163(6 Supplement):S1-S39.
3. Moureau NL, Trick N, Nifong T, Perry C, Kelley C, Carrico R, et al. Vessel health and preservation (Part 1): a new evidence-based approach to vascular access selection and management. J Vasc Access. 2012;13(3):351-6.
4. Anderson NR. Influencing Patient Satisfaction Scores: Prospective One-Arm Study of a Novel Intravenous Catheter System With Retractable Coiled-Tip Guidewire Compared With Published Literature for Conventional Peripheral Intravenous Catheters. Journal of infusion nursing : the official publication of the Infusion Nurses Society. 2016;39(4):201-9.
5. Hallam C, Denton A. Vessel health and preservation 1: minimising the risks of vascular access. Nursing Times. 2020;116(7):22-5.
6. Bahl A, Hijazi M, Chen NW. Vesicant infusates are not associated with ultrasound-guided peripheral intravenous catheter failure: A secondary analysis of existing data. PLoS ONE. 2022;17(1-Jan):e0262793.
7. Faraone V, Aprea P, Spencer TR. AVATAR R: an electronic evidence-based medicine friendly tool for vascular access planning. Minerva medica. 2018;109(4):326-33.
8. Shaw CM, Shah S, Kapoor BS, Cain TR, Caplin DM, Farsad K, et al. ACR Appropriateness Criteria Radiologic Management of Central Venous Access. Journal of the American College of Radiology. 2017;14(11 Supplement):S506-S29.
9. Krein SL, Harrod M, Weston LE, Garlick BR, Quinn M, Fletcher KE, et al. Comparing peripherally inserted central catheter-related practices across hospitals with different insertion models: a multisite qualitative study. BMJ quality & safety. 2021;30(8):628-38.
10. van Loon FHJ, Korsten HHM, Dierick-van Daele ATM, Bouwman ARA. The impact of the catheter to vein ratio on peripheral intravenous cannulation success, a post-hoc analyses. PloS one. 2021;16(5):e0252166.
11. Rabelo-Silva ER, Lourenco SA, Maestri RN, Candido da Luz C, Carlos Pupin V, Bauer Cechinel R, et al. Patterns, appropriateness and outcomes of peripherally inserted central catheter use in Brazil: a multicentre study of 12 725 catheters. BMJ quality & safety. 2022.
12. Moureau NL, Trick N, Nifong T, Perry C, Kelley C, Carrico R, et al. Vessel health and preservation (Part 1): a new evidence-based approach to vascular access selection and management. The journal of vascular access. 2012;13(3):351-6.
13. Ullman AJ, Bernstein SJ, Brown E, Aiyagari R, Doellman D, Faustino EVS, et al. The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC. Pediatrics. 2020;145(Suppl 3):S269-S84.
14. Panebianco NL, Fredette JM, Szyld D, Sagalyn EB, Pines JM, Dean AJ. What you see (sonographically) is what you get: vein and patient characteristics associated with successful ultrasound-guided peripheral intravenous placement in patients with difficult access. Acad Emerg Med. 2009;16(12):1298-303.
15. Moureau NLE. Vessel Health and Preservation: The Right Approach for Vascular Access: Springer Open; 2019.
16. Santolim TQ, Santos LAU, Giovani AMM, Dias VC. The strategic role of the nurse in the selection of IV devices. British journal of nursing (Mark Allen Publishing). 2012;21(21):S28-2.
17. Bechdel BA, Bardman KJ, Machemer C. Developing a Nurse-Driven Vascular Access Device Order Set Using the Electronic Medical Record. Journal of infusion nursing : the official publication of the Infusion Nurses Society. 2022;45(1):20-6.
18. Hallam C, Jackson T, Weston V, Denton A, Hill S, Bodenham A, et al. Development of the UK Vessel Health and Preservation (VHP) framework: a multi-organisational collaborative. Journal of Infection Prevention. 2016;17(2):65-72.
19. Magallon-Pedrera I, Perez-Altozano J, Virizuela Echaburu JA, Beato-Zambrano C, Borrega-Garcia P, de la Torre-Montero JC. ECO-SEOM-SEEO safety recommendations guideline for cancer patients receiving intravenous therapy. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2020;22(11):2049-60.
20. Wells C, Zhang Z, Chan C, Brito A, Kohli-Seth R. Impact of a Peripheral Vascular Access Service on Device Use. Am J Crit Care. 2021;30(4):295-301.
21. Wagner ML, Doellman D, Forlenza KN, Fischer K, Tuncel Kara S, Bradshaw U, et al. Standardizing Preoperative Evaluation for Pediatric Central Venous Access: A Care Algorithm to Improve Safety. Journal of Infusion Nursing. 2020;43(5):262-74.
22. Anderson J, Greenwell A, Louderback J, Polivka BJ, Behr JH. Comparison of Outcomes of Extended Dwell/Midline Peripheral Intravenous Catheters and Peripherally Inserted Central Catheters in Children. JAVA - Journal of the Association for Vascular Access. 2016;21(3):158-64.
23. Barth D, Sanchez A, Thomsen A-M, Garcia A, Malachowski R, Weldon R, et al. Peripheral vascular access for therapeutic plasma exchange: A practical approach to increased utilization and selecting the most appropriate vascular access. Journal of clinical apheresis. 2020;35(3):178-87.
24. Bozaan D, Skicki D, Brancaccio A, Snyder A, Friebe S, Tupps M, et al. Less lumens-less risk: A pilot intervention to increase the use of single-lumen peripherally inserted central catheters. Journal of Hospital Medicine. 2019;14(1):42-6.
25. Chernecky C, Macklin D, Nugent K, Waller JL. Preferences in choosing venous access devices by intravenous and oncology nurses. Journal of Vascular Access Devices. 2003;8(1):35-40.
26. Kelly LJ, Snowden A, Paterson R, Campbell K. Health professionals' lack of knowledge of central venous access devices: the impact on patients. British journal of nursing (Mark Allen Publishing). 2019;28(14):S4-S14.
27. Moureau N, Chopra V. Indications for peripheral, midline and central catheters: summary of the MAGIC recommendations. British journal of nursing (Mark Allen Publishing). 2016;25(8):S15-24.
28. Sowan AK, Beraya AR, Carrola A, Reed C. Effect of a Multimedia Patient Decision Aid to Supplement the Informed Consent Process of a Peripherally Inserted Central Venous Catheter Procedure: Pre-Post Quasi-Experimental Study. JMIR medical informatics. 2018;6(4):e11056.
29. Corti F, Brambilla M, Manglaviti S, Di Vico L, Pisanu MN, Facchinetti C, et al. Comparison of outcomes of central venous catheters in patients with solid and hematologic neoplasms: an Italian real-world analysis. Tumori. 2021;107(1):17-25.
30. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not just an intravenous line: Consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PloS one. 2018;13(2):e0193436.
31. Galloway M. Using benchmarking data to determine vascular access device selection. Journal of infusion nursing : the official publication of the Infusion Nurses Society. 2002;25(5):320-5.
32. Goltz JP, Petritsch B, Kirchner J, Hahn D, Kickuth R. Percutaneous image-guided implantation of totally implantable venous access ports in the forearm or the chest? A patients' point of view. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013;21(2):505-10.
33. Trautner BW, Saint S, Fowler KE, Van J, Rosen T, Colozzi J, et al. What do patients say about their experience with urinary catheters and peripherally inserted central catheters? American journal of infection control. 2019;47(9):1130-4.
34. Walker G, Todd A. Nurse-led PICC insertion: is it cost effective? British journal of nursing (Mark Allen Publishing). 2013;22(19):S9-15.
35. Yagi T, Sakamoto T, Nakai K, Tanizawa M, Okabe T, Hoshikawa N, et al. A Questionnaire-based Assessment of the Anxiety, Satisfaction and Discomfort Experienced by Japanese Cancer Patients during the Use of Central Venous Ports. Internal medicine (Tokyo, Japan). 2016;55(17):2393-9.
36. Harrold K, Martin A, Scarlett C. Proactive PICC placement: evaluating the patient experience. British journal of nursing (Mark Allen Publishing). 2016;25(8):S4-14.
37. Chen YB, Bao HS, Hu TT, He Z, Wen B, Liu FT, et al. Comparison of comfort and complications of Implantable Venous Access Port (IVAP) with ultrasound guided Internal Jugular Vein (IJV) and Axillary Vein/Subclavian Vein (AxV/SCV) puncture in breast cancer patients: a randomized controlled study. BMC cancer. 2022;22(1):248.
38. Chernecky C. Satisfaction versus dissatisfaction with venous access devices in outpatient oncology: a pilot study. Oncology nursing forum. 2001;28(10):1613-6.
39. Babu K, Suresh Babu M, Lokanatha D, Bhat G. Outcomes, cost comparison, and patient satisfaction during long-Term central venous access in cancer patients: Experience from a Tertiary Care Cancer Institute in South India. Indian Journal of Medical and Paediatric Oncology. 2016;37(4):232-8.
40. Blakeley JA, Ribeiro V, Crocker J. Parent satisfaction with education, support, and decision-making regarding their children's central venous access devices. Canadian oncology nursing journal = Revue canadienne de nursing oncologique. 2000;10(1):Aug-13.
41. Burbridge B, Chan IYM, Bryce R, Lim HJ, Stoneham G, Haggag H, et al. Satisfaction and Quality of Life Related to Chemotherapy With an Arm Port: A Pilot Study. Canadian Association of Radiologists journal = Journal l'Association canadienne des radiologistes. 2016;67(3):290-7.
42. Ignatov A, Hoffman O, Smith B, Fahlke J, Peters B, Bischoff J, et al. An 11-year retrospective study of totally implanted central venous access ports: complications and patient satisfaction. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2009;35(3):241-6.
43. Ilkaz N, Iyigun E. Evaluation of Central Venous Catheter Location in Terms of Pain, Comfort and Patient Satisfaction. International Journal of Caring Sciences. 2020;13(1):424-30.
44. Liberale G, El Houkayem M, Viste C, Bouazza F, Moreau M, El Nakadi I, et al. Evaluation of the perceptions and cosmetic satisfaction of breast cancer patients undergoing totally implantable vascular access device (TIVAD) placement. Supportive Care in Cancer. 2016;24(12):5035-40.
45. Marcy P-Y, Schiappa R, Ferrero J-M, Dahlet C, Brenet O, Yazbec G, et al. Patient satisfaction and acceptance of their totally implanted central venous catheter: a French prospective multicenter study. The journal of vascular access. 2017;18(5):390-5.
46. Nagel SN, Teichgraber UKM, Kausche S, Lehmann A. Satisfaction and quality of life: a survey-based assessment in patients with a totally implantable venous port system. European journal of cancer care. 2012;21(2):197-204.
47. Park JY, Lee DI. Experience and Satisfaction of Cancer Patients With a Central Venous Catheter at a Tertiary Hospital in South Korea. Journal of infusion nursing : the official publication of the Infusion Nurses Society. 2020;43(2):97-102.
48. Park K, Jun H, Oh S, Jun HJ, Oh SY. Safety, efficacy, and patient-perceived satisfaction of peripherally inserted central catheters in terminally ill cancer patients: a prospective multicenter observational study. Supportive Care in Cancer. 2016;24(12):4987-92.
49. Kang J, Chen W, Sun W, Ge R, Li H, Ma E, et al. Health-related quality of life of cancer patients with peripherally inserted central catheter: a pilot study. The journal of vascular access. 2017;18(5):396-401.
50. Kim H, Kwon S, Son SM, Jeong E, Kim J-Y. Tailored approach to the choice of long-term vascular access in breast cancer patients. PloS one. 2021;16(7):e0255004.
51. LeVasseur N, Stober C, Ibrahim M, Gertler S, Hilton J, Robinson A, et al. Perceptions of vascular access for intravenous systemic therapy and risk factors for lymphedema in early-stage breast cancer-a patient survey. Current oncology (Toronto, Ont). 2018;25(4):e305-e10.
52. Molloy D, Smith LN, Aitchison T. Cytotoxic chemotherapy for incurable colorectal cancer: living with a PICC-line. Journal of clinical nursing. 2008;17(18):2398-407.
53. Thrush C, Gartside R, Phillipson L. Offering breast cancer patients a choice of device for administration of chemotherapy: a service improvement project. Cancer Nursing Practice. 2020;19(5):16-26.
54. Vermeulin T, Lahbib H, Lottin M, Brifault C, Diot J, Lucas M, et al. Patients' perception and attitude to totally implantable venous access for urologic or digestive cancer: A cross-sectional study. Bulletin du cancer. 2019;106(11):959-68.
55. Voci A, Lee D, Ho E, Crane-Okada R, DiNome M. Impact of port site scar on perception of patients with breast cancer: patient-reported outcomes. Breast cancer research and treatment. 2018;170(3):569-72.
56. Yin L, Li J. Central venous catheter insertion in colorectal cancer patients, PICC or PC? Cancer Management and Research. 2020;12:5813-8.
57. Ryan C, Hesselgreaves H, Wu O, Moss J, Paul J, Dixon-Hughes J, et al. Patient acceptability of three different central venous access devices for the delivery of systemic anticancer therapy: a qualitative study. BMJ open. 2019;9(7):e026077.
58. Qi F, Cheng H, Yuan X, Zhang L. Comparison of PICC and TIVAP in chemotherapy for patients with thyroid cancer. Oncology Letters. 2020;20(2):1657-62.
59. Ying S, Liping Z, Zhulin G, Yanhong D, Liang G. Impact of arm choice for peripherally inserted central catheter (PICC) insertion on patients: a cross-sectional study. Contemporary nurse. 2020;56(1):80-9.
60. McIntosh LA, Walker GM. Patients' experience of portacaths in cystic fibrosis: questionnaire-based study. Archives of disease in childhood. 2015;100(7):659-61.
61. Maurer MH, Beck A, Hamm B, Gebauer B. Central venous port catheters: evaluation of patients' satisfaction with implantation under local anesthesia. The journal of vascular access. 2009;10(1):27-32.
62. Dupont C, Gouya H, Panzo R, Hubert D, Correas J-M, Agrario L, et al. Complications of peripherally inserted central catheters in adults with cystic fibrosis or bronchiectasis. The journal of vascular access. 2015;16(3):245-9.
63. Obadeyi O, Baffoe N, Paxton J. A patient's decision aid for vascular access placement in the emergency department. The journal of vascular access. 2020;21(4):419-25.
64. Biffi R, Orsi F, Pozzi S, Maldifassi A, Radice D, Rotmensz N, et al. No impact of central venous insertion site on oncology patients' quality of life and psychological distress. A randomized three-arm trial. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19(10):1573-80.
65. Bortolussi R, Zotti P, Conte M, Marson R, Polesel J, Colussi A, et al. Quality of Life, Pain Perception, and Distress Correlated to Ultrasound-Guided Peripherally Inserted Central Venous Catheters in Palliative Care Patients in a Home or Hospice Setting. Journal of Pain and Symptom Management. 2015;50(1):118-23.
66. Mangum DS, Verma A, Weng C, Sheng X, Larsen R, Kirchhoff AC, et al. A comparison of central lines in pediatric oncology patients: Early removal and patient centered outcomes. Pediatric blood & cancer. 2013;60(11):1890-5.
67. Cooper AL, Kelly CM, Brown JA. Exploring the patient experience of living with a peripherally inserted central catheter (PICC): A pilot study. Australian Journal of Cancer Nursing. 2017;18(1):Oct-14.
68. Fang S, Yang J, Song L, Jiang Y, Liu Y. Comparison of three types of central venous catheters in patients with malignant tumor receiving chemotherapy. Patient preference and adherence. 2017;11:1197-204.
69. Eclarinal BR, Valdez AP, Ungos PB, Lakim MFHJ, Vher Parago JG. Patients' experience with implantable ports and peripheral lines: Experience of The Brunei Cancer Centre (TBCC). Brunei International Medical Journal (BIMJ). 2014;10(3):138-44.
70. Golding MA, Palmer GD, Fleming GJ. Preference for venepuncture site: should the patient be consulted? Dental update. 2002;29(5):239-43.
71. Goossens GA, Vrebos M, Stas M, De Wever I, Frederickx L. Central vascular access devices in oncology and hematology considered from a different point of view: how do patients experience their vascular access ports? Journal of infusion nursing : the official publication of the Infusion Nurses Society. 2005;28(1):61-7.
72. Kelly LJ, Snowden A. 'Pinholes in my arms': the vicious cycle of vascular access. British journal of nursing (Mark Allen Publishing). 2021;30(14):S4-S13.
73. Krein SL, Saint S, Trautner BW, Kuhn L, Colozzi J, Ratz D, et al. Patient-reported complications related to peripherally inserted central catheters: a multicentre prospective cohort study. BMJ quality & safety. 2019;28(7):574-81.
74. Kreis H, Loehberg CR, Lux MP, Ackermann S, Lang W, Beckmann MW, et al. Patients' attitudes to totally implantable venous access port systems for gynecological or breast malignancies. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2007;33(1):39-43.
75. Kunz-Virk J, Kruger K. Power-injectable totally implantable venous access devices - analysis of success and complication rates of ultrasound-guided implantation and a patient satisfaction survey. VASA Zeitschrift fur Gefasskrankheiten. 2019;48(6):524-30.
76. Larsen E, Keogh S, Marsh N, Rickard C. Experiences of peripheral IV insertion in hospital: a qualitative study. British journal of nursing (Mark Allen Publishing). 2017;26(19):S18-S25.
77. Leonardsen A-CL, Lunde EM, Smith ST, Olsen GL. Patient experiences with peripherally inserted venous catheters- A cross-sectional, multicentre study in Norway. Nursing open. 2020;7(3):760-7.
78. Macklin D, Chernecky C, Nugent K, Waller JL. A collaborative approach to improving patient care associated with vascular access devices. Journal of Vascular Access Devices. 2003;8(2):Aug-13.
79. Paras-Bravo P, Paz-Zulueta M, Santibanez M, Fernandez-de-Las-Penas C, Herrero-Montes M, Caso-Alvarez V, et al. Living with a peripherally inserted central catheter: the perspective of cancer outpatients-a qualitative study. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2018;26(2):441-9.
80. Moller T, Adamsen L. Hematologic patients' clinical and psychosocial experiences with implanted long-term central venous catheter: self-management versus professionally controlled care. Cancer nursing. 2010;33(6):426-35.
81. Piredda M, Biagioli V, Giannarelli D, Incletoli D, Grieco F, Carassiti M, et al. Improving cancer patients' knowledge about totally implantable access port: a randomized controlled trial. Supportive Care in Cancer. 2016;24(2):833-41.
82. Moss JG, Wu O, Bodenham AR, Agarwal R, Menne TF, Jones BL, et al. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): a randomised controlled trial. The Lancet. 2021;398(10298):403-15.
83. Tang T-t, Liu L, Li C-x, Li Y-t, Zhou T, Li H-p, et al. Which is Better for Patients with Breast Cancer: Totally Implanted Vascular Access Devices (TIVAD) or Peripherally Inserted Central Catheter (PICC)? World Journal of Surgery. 2019;43(9):2245-9.
84. McKeown C, Ricciuti A, Agha M, Raptis A, Hou JZ, Farah R, et al. A prospective study of the use of central venous catheters in patients newly diagnosed with acute myeloid leukemia treated with induction chemotherapy. Supportive Care in Cancer. 2022;30(2):1673-9.
85. Bough G, Lambert NJ, Djendov F, Jackson C. Unexpected tunnelled central venous access demise: a single institutional study from the UK. Pediatric surgery international. 2021;37(1):109-17.
86. Ewenstein BM, Valentino LA, Journeycake JM, Tarantino MD, Shapiro AD, Blanchette VS, et al. Consensus recommendations for use of central venous access devices in haemophilia. Haemophilia : the official journal of the World Federation of Hemophilia. 2004;10(5):629-48.
87. Botella-Carretero JI, Carrero C, Guerra E, Valbuena B, Arrieta F, Calanas A, et al. Role of peripherally inserted central catheters in home parenteral nutrition: A 5-year prospective study. Journal of Parenteral and Enteral Nutrition. 2013;37(4):544-9.
88. Cotogni P, Mussa B, Degiorgis C, De Francesco A, Pittiruti M. Comparative Complication Rates of 854 Central Venous Access Devices for Home Parenteral Nutrition in Cancer Patients: A Prospective Study of Over 169,000 Catheter-Days. JPEN Journal of parenteral and enteral nutrition. 2021;45(4):768-76.
89. Huisman-de Waal G, Versleijen M, van Achterberg T, Jansen JB, Sauerwein H, Schoonhoven L, et al. Psychosocial complaints are associated with venous access-device related complications in patients on home parenteral nutrition. JPEN Journal of parenteral and enteral nutrition. 2011;35(5):588-95.
90. Kaji T, Kawano T, Yamada W, Yamada K, Onishi S, Nakame K, et al. The changing profile of safe techniques for the insertion of a central venous catheter in pediatric patients - improvement in the outcome with the experiences of 500 insertions in a single institution. Journal of pediatric surgery. 2016;51(12):2044-7.
91. Konstantinou EA, Karampinis DF, Mitsos AP, Konstantinou MI, Mariolis-Sapsakos T, Kapritsou M, et al. Central vascular catheters versus peripherally inserted central catheters in nurse anesthesia. A perspective within the Greek health system. The journal of vascular access. 2013;14(4):373-8.
92. Magnani C, Calvieri A, Giannarelli D, Espino M, Casale G. Peripherally inserted central catheter, midline, and short midline in palliative care: Patient-reported outcome measures to assess impact on quality of care. The journal of vascular access. 2019;20(5):475-81.
93. Chopra V, Kaatz S, Conlon A, Paje D, Grant PJ, Rogers MAM, et al. The Michigan Risk Score to predict peripherally inserted central catheter-associated thrombosis. Journal of thrombosis and haemostasis : JTH. 2017;15(10):1951-62.
94. Wilson TJ, Stetler Jr WR, Fletcher JJ. Comparison of catheter-related large vein thrombosis in centrally inserted versus peripherally inserted central venous lines in the neurological intensive care unit. Clinical Neurology and Neurosurgery. 2013;115(7):879-82.
95. Scoppettuolo G, Pittiruti M, Pitoni S, Dolcetti L, Emoli A, Mitidieri A, et al. Ultrasound-guided short midline catheters for difficult venous access in the emergency department: a retrospective analysis. International journal of emergency medicine. 2016;9(1):3.
96. Mills CN, Liebmann O, Stone MB, Frazee BW. Ultrasonographically guided insertion of a 15-cm catheter into the deep brachial or basilic vein in patients with difficult intravenous access. Annals of emergency medicine. 2007;50(1):68-72.
97. Clatot F, Fontanilles M, Lefebvre L, Lequesne J, Veyret C, Alexandru C, et al. Randomised phase II trial evaluating the safety of peripherally inserted catheters versus implanted port catheters during adjuvant chemotherapy in patients with early breast cancer. European journal of cancer (Oxford, England : 1990). 2020;126:116-24.
98. Deshpande KS, Hatem C, Ulrich HL, Currie BP, Aldrich TK, Bryan-Brown CW, et al. The incidence of infectious complications of central venous catheters at the subclavian, internal jugular, and femoral sites in an intensive care unit population. Critical Care Medicine. 2005;33(1):13-20.
99. Schults JA, Kleidon TM, Gibson V, Ware RS, Monteagle E, Paterson R, et al. Improving peripheral venous cannula insertion in children: a mixed methods study to develop the DIVA key. BMC health services research. 2022;22(1):220.
100. Angles E, Robin F, Moal B, Roy M, Sesay M, Ouattara A, et al. Pre-operative peripheral intravenous cannula insertion failure at the first attempt in adults: Development of the VENSCORE predictive scale and identification of risk factors. Journal of clinical anesthesia. 2021;75:110435.
101. Civetta G, Cortesi S, Mancardi M, De Pirro A, Vischio M, Mazzocchi M, et al. EA-DIVA score (Enhanced Adult DIVA score): A new scale to predict difficult preoperative venous cannulation in adult surgical patients. The journal of vascular access. 2019;20(3):281-9.
102. de la Torre-Montero J-C, Montealegre-Sanz M, Faraldo-Cabana A, Oliva-Pellicer B, Garcia-Real I, Fenwick M, et al. Venous International Assessment, VIA scale, validated classification procedure for the peripheral venous system. The journal of vascular access. 2014;15(1):45-50.
103. Fiorini J, Piredda M, Zaghini F, Venturini G, Colella S, Conti F, et al. Vessel health and preservation: Development and validation of a proactive instrument. Collegian. 2021;28(5):528-33.
104. Moondeep K, Anil K, Anshul D. Effect of Moist Heat Therapy on the Visibility and Palpability of Peripheral Veins Before Peripheral Venous Cannulation among Patients Undergoing Intravenous Cannulation- A Quasi Experimental Study. International Journal of Nursing Care. 2020;8(2):Oct-14.
105. O'Neill MB, Dillane M, Hanipah NF. Validating the difficult intravenous access clinical prediction rule. Pediatric Emergency Care. 2012;28(12):1314-6.
106. Salleras-Duran L, Fuentes-Pumarola C, Ballester-Ferrando D, Congost-Devesa L, Delclos-Rabassa J, Fontova-Almato A. Development, Diagnostic Sensitivity, and Prognostic Accuracy of the Adult-Difficult Venous Catheterization Scale for Emergency Departments. Journal of emergency nursing. 2020;46(6):827-37.e2.
107. Shaukat H, Neway B, Breslin K, Watson A, Poe K, Boniface K, et al. Utility of the DIVA score for experienced emergency department technicians. British journal of nursing (Mark Allen Publishing). 2020;29(2):S35-S40.
108. Sou V, McManus C, Mifflin N, Frost SA, Ale J, Alexandrou E. A clinical pathway for the management of difficult venous access. BMC Nurs. 2017;16:64.
109. Yen K, Riegert A, Gorelick MH. Derivation of the DIVA score: a clinical prediction rule for the identification of children with difficult intravenous access. Pediatr Emerg Care. 2008;24(3):143-7.
110. Wells S. Venous access in oncology and haematology patients: Part two. Nurs Stand. 2008;23(1):35-42.
111. Sharp R, Carr P, Childs J, Scullion A, Young M, Flynn T, et al. Catheter to vein ratio and risk of peripherally inserted central catheter (PICC)-associated thrombosis according to diagnostic group: a retrospective cohort study. BMJ Open. 2021;11(7):e045895.
112. Cochrane HK, Henwood PC, Platz E, Koskenoja V, Landry A, Frasure SE, et al. A randomized trial of ultrasound-guided peripheral IV catheter placement in difficult access patients using a guidewire approach. The American journal of emergency medicine. 2020;38(1):122-6.
113. Doggett BM, Session-Augustine N, Roig J, Strunk M, Valiyaparambil S, Sarode R, et al. Single-needle: an effective alternative to dual-needle peripheral access in therapeutic plasma exchange. Journal of clinical apheresis. 2019;34(1):21-5.
114. Fabiani A, Dreas L, Sanson G. Ultrasound-guided deep-arm veins insertion of long peripheral catheters in patients with difficult venous access after cardiac surgery. Heart & lung : the journal of critical care. 2017;46(1):46-53.
115. Putensen D, Leverett D, Patel B, Rivera J. Is peripheral access for apheresis procedures underutilized in clinical practice?-A single centre experience. Journal of clinical apheresis. 2017;32(6):553-9.
116. Qin KR, Pittiruti M, Nataraja RM, Pacilli M. Long peripheral catheters and midline catheters: Insights from a survey of vascular access specialists. The journal of vascular access. 2021;22(6):905-10.
117. Santucci SE, Trerotola SO. Nursing-Placed Midline Catheters and Ultrasound- Guided Peripheral IVs Promote More Appropriate Catheter Selection. Journal of the Association for Vascular Access. 2020;25(2):14-22.
118. Stowell JR, Rigdon D, Colglazier R, Filler L, Orosco D, Connell M, et al. Risk of contrast extravasation with vascular access in computed tomography. Emergency radiology. 2020;27(3):253-8.
119. Ramer L, Hunt P, Ortega E, Knowlton J, Briggs R, Hirokawa S. Effect of Intravenous (IV) Assistive Device (VeinViewer) on IV Access Attempts, Procedural Time, and Patient and Nurse Satisfaction. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses. 2016;33(4):273-81.
120. Buisson M, Leguelinel G, Bastide S, Beregi JP, Kinowski JM, Frandon J, et al. A new clinical approach to improve the appropriate use of peripherally inserted central catheters: a prospective study. European journal of hospital pharmacy : science and practice. 2021;28(Suppl 2):e134-e9.
121. Fearonce G, Faraklas I, Saffle JR, Cochran A. Peripherally inserted central venous catheters and central venous catheters in burn patients: A comparative review. Journal of Burn Care and Research. 2010;31(1):31-5.
122. Murayama R, Oyama H, Abe-Doi M, Masamoto Y, Kashiwabara K, Tobe H, et al. Safety verification of a new peripheral intravenous catheter placed in the upper arm vein for administration of drugs with high irritant potential. Drug discoveries & therapeutics. 2022.
123. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: A randomized controlled trial. Pediatrics. 2021;147(2):e2020000877.
124. Idemoto BK, Rowbottom JR, Reynolds JD, Hickman Jr RL. The accucath intravenous catheter system with retractable coiled tip guidewire and conventional peripheral intravenous catheters: A prospective, randomized, controlled comparison. JAVA - Journal of the Association for Vascular Access. 2014;19(2):94-102.
125. Morgeli R, Schmidt K, Neumann T, Kruppa J, Fohring U, Hofmann P, et al. A comparison of first-attempt cannulation success of peripheral venous catheter systems with and without wings and injection ports in surgical patients-a randomized trial. BMC anesthesiology. 2022;22(1):88.
126. Seetharam AM, Raju U, Suresh K. A randomized controlled study to compare first stick success with Instaflash technology: The FIRSST study. The journal of vascular access. 2022:1.13E+6.
127. Simhi E, Kachko L, Bruckheimer E, Katz J. A vein entry indicator device for facilitating peripheral intravenous cannulation in children: a prospective, randomized, controlled trial. Anesthesia and analgesia. 2008;107(5):1531-5.
128. van Loon FH, Timmerman R, den Brok GP, Korsten EH, Dierick-van Daele AT, Bouwman AR. The impact of a notched peripheral intravenous catheter on the first attempt success rate in hospitalized adults: Block-randomized trial. The journal of vascular access. 2022;23(2):295-303.
129. Ozsarac M, Dolek M, Sarsilmaz M, Sever M, Sener S, Kiyan S, et al. The effect of cannula material on the pain of peripheral intravenous cannulation in the emergency department: A prospective, randomized controlled study. Turkiye Acil Tip Dergisi. 2012;12(4):151-6.
130. Matthews R, Gavin NC, Marsh N, Marquart-Wilson L, Keogh S. Peripheral intravenous catheter material and design to reduce device failure: A systematic review and meta-analysis. Infect Dis Health. 2023;28(4):298-307.
131. Tamura A, Kato K, Kamata M, Suzuki T, Suzuki M, Nakayama M, et al. Selection of peripheral intravenous catheters with 24-gauge side-holes versus those with 22-gauge end-hole for MDCT: A prospective randomized study. European journal of radiology. 2017;87:8-Dec.
132. Chopra V, Kaatz S, Swaminathan L, Boldenow T, Snyder A, Burris R, et al. Variation in use and outcomes related to midline catheters: results from a multicentre pilot study. BMJ quality & safety. 2019;28(9):714-20.
133. Gilardi E, Giannuzzi R, WoldeSellasie K, Piano A, Pittiruti M, Scoppettuolo G. Mini-midline in difficult intravenous access patients in emergency department: A prospective analysis. J Vasc Access. 2020;21(4):449-55.
134. Seo H, Altshuler D, Dubrovskaya Y, Nunnally ME, Nunn C, Ello N, et al. The Safety of Midline Catheters for Intravenous Therapy at a Large Academic Medical Center. Annals of Pharmacotherapy. 2020;54(3):232-8.
135. Tao F, Wang X, Liu J, Li J, Sui F. Perioperative application of midline catheter and PICC in patients with gastrointestinal tumors. Journal of BUON. 2019;24(6):2546-52.
136. Xu T, Kingsley L, DiNucci S, Messer G, Jeong J-H, Morgan B, et al. Safety and utilization of peripherally inserted central catheters versus midline catheters at a large academic medical center. American Journal of Infection Control. 2016;44(12):1458-61.
137. Kleidon TM, Schults JA, Wainwright C, Mihala G, Gibson V, Saiyed M, et al. Comparison of midline catheters and peripherally inserted central catheters to reduce the need for general anesthesia in children with respiratory disease: A feasibility randomized controlled trial. Paediatric anaesthesia. 2021;31(9):985-95.
138. Lescinskas EH, Trautner BW, Saint S, Colozzi J, Evertsz K, Chopra V, et al. Use of and patient-reported complications related to midline catheters and peripherally inserted central catheters. Infection control and hospital epidemiology. 2020;41(5):608-10.
139. Alexandrou E, Ramjan LM, Spencer T, Frost SA, Salamonson Y, Davidson PM, et al. The use of midline catheters in the adult acute care setting - Clinical implications and recommendations for practice. JAVA - Journal of the Association for Vascular Access. 2011;16(1):35-8.
140. Benvenuti S, Ceresoli R, Boroni G, Parolini F, Porta F, Alberti D. Use of peripherally inserted central venous catheters (PICCs) in children receiving autologous or allogeneic stem-cell transplantation. Journal of Vascular Access. 2018;19(2):131-6.
141. Bui S, Babre F, Hauchecorne S, Christoflour N, Ceccato F, Boisserie-Lacroix V, et al. Intravenous peripherally-inserted central catheters for antibiotic therapy in children with cystic fibrosis. Journal of Cystic Fibrosis. 2009;8(5):326-31.
142. Park EJ, Park K, Kim JJ, Oh SB, Jung KS, Oh SY, et al. Safety, Efficacy, and Patient Satisfaction with Initial Peripherally Inserted Central Catheters Compared with Usual Intravenous Access in Terminally Ill Cancer Patients: A Randomized Phase II Study. Cancer Res Treat. 2021;53(3):881-8.
143. Yamada R, Morita T, Yashiro E, Otani H, Amano K, Tei Y, et al. Patient-reported usefulness of peripherally inserted central venous catheters in terminally ill cancer patients. Journal of pain and symptom management. 2010;40(1):60-6.
144. Xiao M-F, Xiao C-Q, Li J, Dai C, Fan Y-Y, Cao H-J, et al. Subcutaneous tunneling technique to improve outcomes for patients undergoing chemotherapy with peripherally inserted central catheters: a randomized controlled trial. The Journal of international medical research. 2021;49(4):3.00061E+15.
145. Xiao W, Lin Q, Chen S, Li S, Lin C, Su S, et al. Catheterization of PICC through a superficial femoral vein for patients with superior vena cava syndrome using ECG positioning and ultrasound-guided technologies. The journal of vascular access. 2021:1.12973E+16.
146. Jeon E-Y, Cho YK, Yoon DY, Hwang JH. Which arm and vein are more appropriate for single-step, non-fluoroscopic, peripherally inserted central catheter insertion? The journal of vascular access. 2016;17(3):249-55.
147. Salari M, Sasani MR, Masjedi M, Pourali A, Aghazadeh S. The association of diameter and depth of internal jugular and subclavian veins with hand dominancy. Electronic physician. 2018;10(7):7115-9.
148. Paquet F, Boucher LM, Valenti D, Lindsay R. Impact of arm selection on the incidence of picc complications: Results of a randomized controlled trial. Journal of Vascular Access. 2017;18(5):408-14.
149. Martins FTM, Carvalho EC. Patients’ perception regarding the use of a long-term catheter. Rev Esc Enferm USP 2008;42(3):526–31.
150. Lefebvre L, Noyon E, Georgescu D, Proust V, Alexandru C, Leheurteur M, et al. Port catheter versus peripherally inserted central catheter for postoperative chemotherapy in early breast cancer: a retrospective analysis of 448 patients. Support Care Cancer. 2016;24(3):1397-403.
151. Minichsdorfer C, Fureder T, Mahr B, Berghoff AS, Heynar H, Dressler A, et al. A Cross-Sectional Study of Patients' Satisfaction With Totally Implanted Access Ports. Clinical journal of oncology nursing. 2016;20(2):175-80.
152. Patel GS, Jain K, Kumar R, Strickland AH, Pellegrini L, Slavotinek J, et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer. 2014;22(1):121-8.
153. Povoski SP, Zaman SA. Selective use of preoperative venous duplex ultrasound and intraoperative venography for central venous access device placement in cancer patients. Ann Surg Oncol. 2002;9(5):493-9.
154. Silvestri V, Nerini L, Missio G, Masini M, Faggi S, Gori A, et al. Levels of anxiety and pain during chemotherapy with peripheral versus central vascular access: An experimental evaluation. Journal of Vascular Access. 2004;5(4):147-53.
155. Singh KR, Agarwal G, Nanda G, Chand G, Mishra A, Agarwal A, et al. Morbidity of chemotherapy administration and satisfaction in breast cancer patients: a comparative study of totally implantable venous access device (TIVAD) versus peripheral venous access usage. World journal of surgery. 2014;38(5):1084-92.
156. Chand R, Sertic M, Nemec R, Tomlin K, Connolly B. Use of Vascular Ports for Long-Term Apheresis in Children. Journal of vascular and interventional radiology : JVIR. 2015;26(11):1669-72.e1.
157. Michell H, Nezami N, Morris C, Hong K. Dual-chambered venous access port as alternative access for extracorporeal apheresis therapy. Journal of Vascular Access. 2021;22(2):173-7.
158. Hooda B, Lalani G, Fadoo Z, Billoo G. Implantable port devices are catheters of choice for administration of chemotherapy in pediatric oncology patients - A clinical experience in Pakistan. Recent Advances in Clinical Oncology. 2008;1138:43-6.
159. Kehagias E, Tsetis D. The Arm-to-Chest Tunneling technique: A modified technique for arm placement of implantable ports or central catheters. The journal of vascular access. 2019;20(6):771-7.
160. Burbridge B, Goyal K. Quality-of-life assessment: arm TIVAD versus chest TIVAD. The journal of vascular access. 2016;17(6):527-34.
161. Busch JD, Vens M, Mahler C, Herrmann J, Adam G, Ittrich H. Complication Rates Observed in Silicone and Polyurethane Catheters of Totally Implanted Central Venous Access Devices Implanted in the Upper Arm. Journal of Vascular and Interventional Radiology. 2017;28(8):1177-83.
162. Campbell WB, Elworthy S, Peerlinck I, Vanslembroek K, Bangur R, Stableforth D, et al. Sites of implantation for central venous access devices (ports): a study of the experiences and preferences of patients. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2004;28(6):642-4.
163. Fonseca IY, Krutman M, Nishinari K, Yazbek G, Teivelis MP, Bomfim GA, et al. Brachial insertion of fully implantable venous catheters for chemotherapy: complications and quality of life assessment in 35 patients. Einstein (Sao Paulo, Brazil). 2016;14(4):473-9.
164. Herd F, Miller T, van Delft FW, Gabra HO. The peripheral portacath provides safe and convenient venous access in pediatric and adolescent patients. Journal of pediatric surgery. 2019;54(7):1449-52.
165. Marcy PY, Lacout A, Thariat J, Figl A, Merckx J. Ultrasound-guided arm ports: Indications, techniques, and management. JAVA - Journal of the Association for Vascular Access. 2015;20(1):26-31.
166. Burbridge B, Lim H, Dwernychuk L, Le H, Asif T, Sami A, et al. Comparison of the Quality of Life of Patients with Breast or Colon Cancer with an Arm Vein Port (TIVAD) Versus a Peripherally Inserted Central Catheter (PICC). Current oncology (Toronto, Ont). 2021;28(2):1495-506.
167. Nas OF, Hacikurt K, Kaya A, Dogan N, Sanal B, Ozkaya G, et al. Choosing the appropriate side for subcutaneous port catheter placement in patients with mastectomy: ipsilateral or contralateral? Radiol Med. 2017;122(6):472-8.
168. Yang S-S, Ahn MS. A Comparison Between Upper Arm and Chest for Optimal Site of Totally Implanted Venous Access Ports in Patients with Female Breast Cancer. Annals of vascular surgery. 2018;50:128-34.
169. Kehagias E, Tsetis D. The L-shaped tunneling technique: a modified technique facilitating a more discreet implantable port positioning. The journal of vascular access. 2016;17(2):195-9.
170. Cil BE, Ocal O, Eldem FG, Peynircioglu B, Balkanci F. Trapezius Port Placement in Patients with Breast Cancer: Long-Term Follow-up and Quality-of-Life Assessment. Journal of vascular and interventional radiology : JVIR. 2019;30(1):69-73.
171. Hussain S, Khan RA, Iqbal M, Shafiq M. A comparative study of supraclavicular versus infraclavicular approach for central venous catheterization. Anaesthesia, Pain and Intensive Care. 2011;15(1):13-6.
172. Toro A, Mannino M, Cappello G, Celeste S, Cordio S, Di Carlo I. Totally implanted venous access devices implanted in the saphenous vein. Relation between the reservoir site and comfort/discomfort of the patients. Annals of vascular surgery. 2012;26(8):1127.e9-.e13.
173. Cavallaro G, Iorio O, Iossa A, De Angelis F, Avallone M, Massaro M, et al. A prospective evaluation on external jugular vein cut-down approach for TIVAD implantation. World journal of surgical oncology. 2015;13:243.
174. Han L, Zhang J, Deng X, Kong X, Yang C, Peng L, et al. Totally implantable venous access ports: A prospective randomized study comparing subclavian and internal jugular vein punctures in children. Journal of Pediatric Surgery. 2021;56(2):317-23.
175. Plumhans C, Mahnken AH, Ocklenburg C, Keil S, Behrendt FF, Gunther RW, et al. Jugular versus subclavian totally implantable access ports: catheter position, complications and intrainterventional pain perception. European journal of radiology. 2011;79(3):338-42.
176. Menekli T, Yaprak B, Dogan R. The Effect of Virtual Reality Distraction Intervention on Pain, Anxiety, and Vital Signs of Oncology Patients Undergoing Port Catheter Implantation: A Randomized Controlled Study. Pain management nursing : official journal of the American Society of Pain Management Nurses. 2022.
177. Bortolussi R, Zotti P, Matovic M, Morabito A, Bertuzzi C, Caserta M, et al. A phase II study on the efficacy and safety of procedural analgesia with fentanyl buccal tablet in cancer patients for the placement of indwelling central venous access systems. Supportive Care in Cancer. 2016;24(4):1537-43.
178. Celebioglu EC, Bilgic MS. Ultrasound-guided supraclavicular nerve block for implantable port catheters: Does it show a significant difference in pain control? The journal of vascular access. 2022;23(2):206-11.
179. Chang D-H, Hiss S, Herich L, Becker I, Mammadov K, Franke M, et al. Implantation of venous access devices under local anesthesia: patients' satisfaction with oral lorazepam. Patient preference and adherence. 2015;9:943-9.
180. Mejri N, Haj Mansour M, Labidi S, El Benna H, Afrit M, Boussen H. Acute Pain Related to Vascular Access Port Implantation in Adult Cancer Patients: A Prospective Assessment. JAVA - Journal of the Association for Vascular Access. 2017;22(4):188-92.
181. Piskin O, Altinsoy B, Baytar C, Aydin BG, Okyay D, Kucukosman G, et al. The effect of PECS-1 block on postoperative pain in total implantable venous access port catheter (TIVAP) insertion. The journal of vascular access. 2021:1.12973E+16.
182. Sovinz P, Urban C, Lackner H, Kerbl R, Schwinger W, Dornbusch H. Tunneled femoral central venous catheters in children with cancer. Pediatrics. 2001;107(6):E104.
183. Zhai S, Zhao Q, Feng Y, Yu Y, Zhou L, Cui T, et al. Placement of tunneled cuffed vascular catheter through superior vena cava puncture. Journal of vascular surgery Venous and lymphatic disorders. 2017;5(4):547-52.
184. Moreiras-Plaza M, Albo C, Ares C. Efficacy and safety of femoral vascular access for peripheral blood stem cell (PBSC) collection. Bone Marrow Transplantation. 2004;33(3):347-50.
185. Ritchie M, Kelly LJ, Moss J, Paul J, Shaw R. Exploring attitudes towards a randomised controlled trial of venous access devices - a nested pre-trial qualitative study. The journal of vascular access. 2015;16(5):407-12.
186. Corcuera Martinez MI, Aldonza Torres M, Diez Revilla AM, Maali Centeno S, Maneru Oria A, Elizari Roncal I, et al. Impact assessment following implementation of a vascular access team. The journal of vascular access. 2022;23(1):135-44.
187. DeVries M, Sleweon T. Bridging the Gap: Introduction of an Antimicrobial Peripherally Inserted Central Catheter (PICC) in Response to High PICC Central Line-Associated Bloodstream Infection Incidence. Journal of the Association for Vascular Access. 2021;26(2):7-Dec.
188. Oakley C, Wright E, Ream E. The experiences of patients and nurses with a nurse-led peripherally inserted central venous catheter line service. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2000;4(4):207-18.
189. Ray-Barruel G, Cooke M, Chopra V, Mitchell M, Rickard CM. The I-DECIDED clinical decision-making tool for peripheral intravenous catheter assessment and safe removal: a clinimetric evaluation. BMJ Open. 2020;10(1):e035239.
190. Piredda M, Migliozzi A, Biagioli V, Carassiti M, De Marinis MG. Written Information Improves Patient Knowledge About Implanted Ports. Clinical journal of oncology nursing. 2016;20(2):E28-33.
191. Ehrhardt BS, Givens KEA, Lee RC. Making It Stick: Developing and Testing the Difficult Intravenous Access (DIVA) Tool. The American journal of nursing. 2018;118(7):56-62.
192. Wojnar DG, Beaman ML. Peripherally inserted central catheter: Compliance with evidence-based indications for insertion in an inpatient setting. Journal of Infusion Nursing. 2013;36(4):291-6.
193. Panter M, Olson DM, Stutzman SE, Aiyagari V. A Decision Algorithm Is Not Superior to Clinician Judgment to Determine Need for Peripheral vs Central Venous Catheterization. The Journal of neuroscience nursing : journal of the American Association of Neuroscience Nurses. 2019;51(3):129-33.
194. To Y-Y, Sutcliffe KJ, Hoen HM, Swaney RA, Nicholls JD, Crocenzi TS. Comparison of local reactions to oxaliplatin infusions by peripheral versus central venous administration. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2016;22(2):256-60.
195. Son BG, Kim MJ, Park MH, Kim K, Kim J, Kim S-Y, et al. Two Small Intravenous Catheters for High-Rate Contrast Medium Injection for Computed Tomography in Patients Lacking Superficial Veins to Accommodate a Large Catheter. Korean journal of radiology. 2018;19(3):489-97.
196. Johnson PT, Christensen G, Lai H, Eng J, Fishman EK. Catheter insertion for intravenous (IV) contrast infusion in multidetector-row computed tomography (MDCT): Defining how catheter caliber selection affects procedure of catheter insertion, IV contrast infusion rate, complication rate, and MDCT image quality. Journal of Computer Assisted Tomography. 2014;38(2):281-4.
197. Periard D, Monney P, Waeber G, Zurkinden C, Mazzolai L, Hayoz D, et al. Randomized controlled trial of peripherally inserted central catheters vs. peripheral catheters for middle duration in-hospital intravenous therapy. Journal of thrombosis and haemostasis : JTH. 2008;6(8):1281-8.
198. Jain S, Shukla S, Talati S, Parikh S, Bhatt S, Maka V. A retrospective study of central venous catheters GCRI experience. Indian Journal of Medical and Paediatric Oncology. 2013;34(4):238-41.
199. Johansson E, Engervall P, Bjorvell H, Hast R, Bjorkholm M. Patients' perceptions of having a central venous catheter or a totally implantable subcutaneous port system-results from a randomised study in acute leukaemia. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2009;17(2):137-43.
200. Crocoli A, Tornesello A, Pittiruti M, Barone A, Muggeo P, Inserra A, et al. Central venous access devices in pediatric malignancies: a position paper of Italian Association of Pediatric Hematology and Oncology. Journal of Vascular Access. 2015;16(2):130-6.
201. Ahmadi J, Izadyar M, Ashjaei B, Klantari M, Nahvi H, Joodi M, et al. Study of advantages and disadvantages of totally implantable venous access device. Acta Medica Iranica. 2006;44(3):199-202.
202. Hitz F, Klingbiel D, Omlin A, Riniker S, Zerz A, Cerny T. Athrombogenic coating of long-term venous catheter for cancer patients: A prospective, randomised, double-blind trial. Annals of Hematology. 2012;91(4):613-20.
203. Carr PJ, Higgins NS, Cooke ML, Rippey J, Rickard CM. Tools, Clinical Prediction Rules, and Algorithms for the Insertion of Peripheral Intravenous Catheters in Adult Hospitalized Patients: A Systematic Scoping Review of Literature. Journal of hospital medicine. 2017;12(10):851-8.
204. Carr PJ, Rippey JCR, Cooke ML, Higgins NS, Trevenen ML, Foale A, et al. Derivation of a clinical decision-making aid to improve the insertion of clinically indicated peripheral intravenous catheters and promote vessel health preservation. An observational study. PloS one. 2019;14(3):e0213923.
205. Hawkins T, Greenslade JH, Suna J, Williams J, Rickard CM, Jensen M, et al. Peripheral Intravenous Cannula Insertion and Use in the Emergency Department: An Intervention Study. Acad Emerg Med. 2018;25(1):26-32.
206. Chick JFB, Reddy SN, Chen JX, Sammarco T, Chittams J, Trerotola SO. A randomized comparison between an intravenous catheter system with a retractable guidewire and conventional intravenous catheters. The journal of vascular access. 2017;18(6):530-4.
207. Anderson NR. Midline catheters: the middle ground of intravenous therapy administration. Journal of infusion nursing : the official publication of the Infusion Nurses Society. 2004;27(5):313-21.
208. Nickel B. Does the Midline Peripheral Intravenous Catheter Have a Place in Critical Care? Critical Care Nurse. 2021;41(6):e1-e21.
209. Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, Scoppettuolo G. A prospective, randomized comparison of three different types of valved and non-valved peripherally inserted central catheters. Journal of Vascular Access. 2014;15(6):519-23.

