Section 2: Vessel Health and Preservation
Version 2: January 2024
In addition to the studies identified in this review, the Australian Commission on Safety and Quality in Health Care will be referenced as our national standard for peripheral intravenous cannula (PIVC) management.
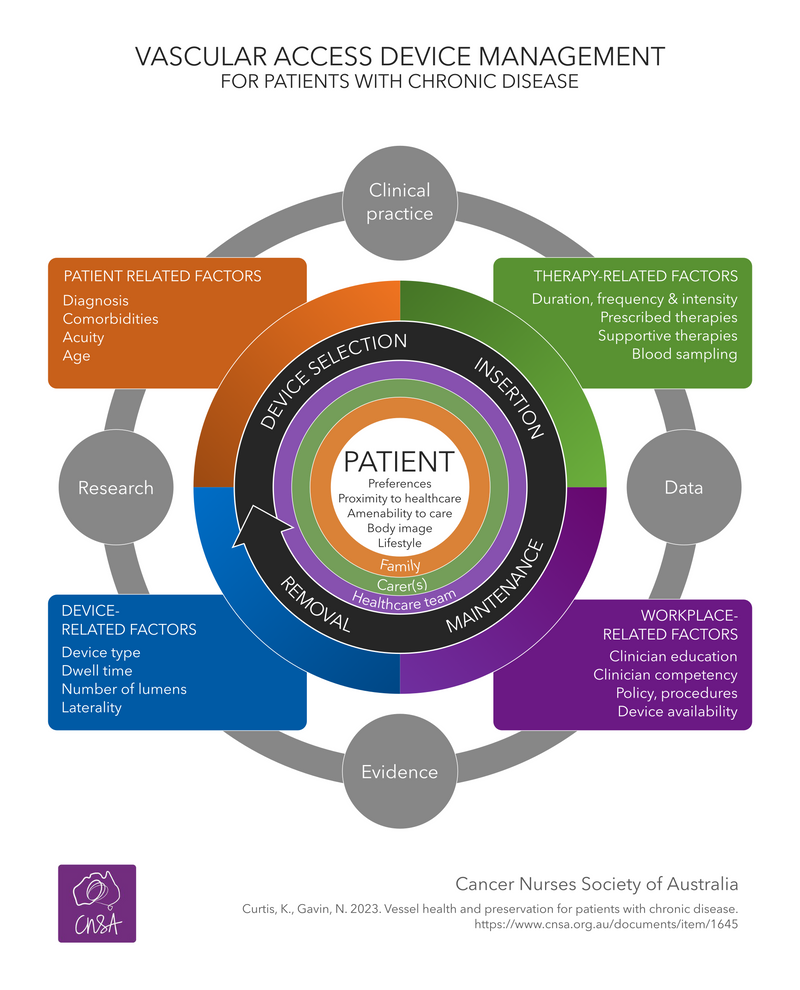
Systematic integration of current evidence and local data, with local knowledge and expertise in a workplace is used to improve clinical care and positively impact patient outcomes (1). This means selection of the most appropriate VAD for the individual patient and prescribed therapy, maximising first insertion success, reducing VAD-related complications and premature removals outcomes for patients with cancer (2, 3).
The VAD&IT SPN in 2025 will be to undertaking two projects, (1) to develop an algorithm for a standardised, evidence-based approach to device selection for patients with chronic diseases such as cancer, and (2) importantly, to co-design patient resources to facilitate informed patient involvement in the decision-making process.
COLLABORATION & ENDORSEMENT
Collaboration between key professional bodies and standardisation of evidence-based clinical practice is key to reducing the risk vascular access related complications and premature removal.
These guidelines have been endorsed by the following organisations:

These guidelines answer three common questions from clinical practice:
Kerrie Curtis, Nurse Consultant, B(Nurs), M(Nurs-Cancer/Palliative Care), PhD(Candidate)
Nicole Gavin, RN, PhD, Cancer Care Nurse Researcher, PhD, B(Sci-Hon), M (AdvPr-HCR)
Carmel O’Kane, Nurse Practitioner, M(Nurs), GDip(Project Mgt), GDip(Mgt), GCert. (Infectious Diseases)
Elena Tarasenko, Clinical Nurse Specialist, M(AdvPracNurs)
Carolyn Meredith, Regional Nurse Educator, B(Nurs), GCert(Cancer, Haematology Nursing), GCert (HlthProdEd)
Susan Richardson, Clinical Nurse Consultant, B(Nurs), M(NursSci-Oncology), GCert(ClinEd)
Kate Schmetzer, Clinical Nurse Educator, B(Nurs), M(Ed), GDip(AdvClinNurs)
Jane Kelly, Clinical Nurse Consultant, B(Nurs), GCert(CancerCare)
Trudie Lawson, Registered Nurse, M(ClinEd), GCert(Cancer Care), B(Nurs)
Emily Larsen, Registered Nurse, B(HlthSci), GDip(HlthRes), PhD(Candidate)
Chun Hei Jason Lam, Registered Nurse, BN(AdvStud), GCert(CanHaemNurs)
Fiona Fuller, Registered Nurse, B(Nurs), GCert(Cancer Care), Cert(Prostate Cancer)
Geoff Hill, Specialist Medical Librarian
SEARCH STRATEGY
A systematic review methodology was utilized to identify, search and analyse current literature. Refer here for more details.
Clinical questions
Clinical questions using the population, intervention, comparator, outcome (PICO) format informed the search strategy:
- What is vessel health and preservation?
P: all patient cohorts
I: vein health
C: venous depletion
O: strategy (algorithm, decision pathway) for vessel preservation
- How does the clinical team decide what the most appropriate vascular access device is for the individual patient and the prescribed therapies with the aim of preserving vessel health?
P: all patient cohorts
I: vascular access device selection process
C: difficult intravenous access
O: preservation of vessel health
- How is the patient included, informed, and actively involved in the decision-making process for the most appropriate vascular access device for them & their prescribed therapies to preserve their vessel health?
P: all patient cohorts
I: informed patient involved in device selection or experience of VADs
C: patient impact when not involved
O: decision by the healthcare team including the patient
In collaboration with a medical librarian, a search was established for each question.
Inclusion
- Study designs: interventional and observational studies - randomised controlled trials, cohort, expert delphi or consensus if VHP or device selection related, algorithm or tool for device selection
- Age group: adults, adolescent and paediatric patients
- Patient cohort: all patient population (not just oncology)
- Vascular access devices: peripheral intravenous cannulas (PIVC), peripherally inserted central catheters (PICC), centrally inserted central catheters (CICC), tunnelled cuffed-CICCs (tc-CICC), totally implantable venous access devices (TIVADs), apheresis-CICCs (A-CICC) and tunnelled cuffed A-CICC (tc-A-CICC)
- Topic: 1. VAD selection and assistive technology for first time success including for difficult intravenous access 2. Patient experience, inclusion, informed decision and education
Exclusion
- Study designs: case studies, conference abstracts, education literature, letter to editor, study protocols, guidelines
- Age groups: neonates, infants
- Vascular access devices: Haemodialysis-CICCs, arterial, midline and intraosseus devices
- Topic: Study outcomes are VAD complications only and not prolonged outcome of VHP. Assumption based on a large body of evidence is that ultrasound guided PIVC and CVAD insertion has positive outcomes, so studies where the outcome is related to US use to prevent VAD complications or comparison of US versus traditional insertion was excluded.
LITERATURE SEARCH
Literature search
The search was run in July 2022 in Medline, Embase and CINAHL (Figure 1)

Figure 1
Summary of evidence
Characteristics of studies
Of the final 378 studies, the majority were from Europe (n=132), followed by the USA (n=116), Australia (n=24), China (n=20), multinational (n=14), Turkey (n=12), Canada and India (n=10 each), Japan (n=9), Korea (n=7), Brazil and South Korea (n=4), Iran and Pakistan (n=2) and Argentina, Austria, Brunei, Egypt, Indonesia, Israel, Malaysia, Saudi Arabia, Singapore, South Africa and Tunisia (n=1 each). Study designs included randomised controlled trials (n=XX), observational studies (n=XX), surveys / questionnaires (n=XX) and expert opinion/Delphi studies (n=XX). Interestingly as this is an emerging subject, the number of studies is steadily increasing, and a notable increase around the times of two key publications by Moureau and colleagues (2012) and Hallam and colleagues (2016). (Figure 2)

Figure 2
WITH THANKS TO
These guidelines were made possible thanks to an unrestricted educational grant from ICU Medical. We are grateful for their leadership and contribution.
