Vascular access devices for people with cancer
The CNSA Vascular Access Device and Infusion Therapy Specialist Practice Network (VAD&IT SPN) are expert cancer nurses and researchers dedicated to improving the clinical management of vascular access devices through integration of current evidence, local and system level data, multidisciplinary and collaborative clinical expertise to positively impact our patient’s vascular access experiences and outcomes.
The VAD&IT SPN are leading several projects:
-
Revision of the CNSA Vascular Access Guidelines
-
Annual pre-Congress symposium on contemporary vascular access topics
-
Active communication and collaboration with cancer nurses on the CNSA Sosido platform

The CNSA Vascular Access Devices: Evidence-Based Clinical Practice Guidelines are recommendations for the safe, effective, and efficient management of vascular access devices (VAD) for patients with cancer. They incorporate central venous access devices and peripheral intravenous cannulas for adult and paediatric patient populations. Topics currently covered include Patency, occlusion prevention and management along with Vessel Health and Preservation.
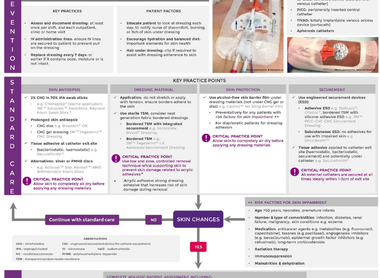
Skin management is complex for patients with cancer related to the impact of the disease process and adverse effects of prescribed therapies. Maintenance of skin health and prevention of skin impairment is key. Evidence-based management incorporating standard care, alternative practices and products is summarised in the CNSA Skin Management Algorithm.
The guidelines are being updated sequentially by topic. Patency, occlusion prevention and management details current evidence and recommendations for eight common clinical questions. The second topic Vessel Health and Preservation incorporates device selection and difficult intravenous access through an oncology lens was launched in March 2024. The next topic will address the complexity of dressing management and securement.
Collaboration between key professional bodies and standardisation of evidence-based clinical practice is key to reducing the risk vascular access related complications and premature removal.
These guidelines have been endorsed by the following organisations:

THE CALL TO ACTION
The CNSA VAD&IT SPN states:
► We need to improve our patient’s outcomes.
Collaborative engagement of the multidisciplinary team including the patient, based on the best, currently available evidence, and informed by local and system level data is required to improve clinical practice. The aim is to improve our patient’s outcomes and experiences by reducing the risk of complications and premature removal of peripheral intravenous cannulas and central venous access devices.
► We need data.
A minimum data set in each cancer centre is required to contribute to a national vascular access device registry, to assist with answering current clinical questions and to inform future research. This would include patient, device, insertion, management, complication and removal data.
► We need more research.
Good quality research is required to guide clinical practice for vascular access device management for patients with cancer.
If you are interested in joining this dynamic group of cancer nurses and researchers, please get in touch.




